Why MDA’s Kickstart Program Is a Game-changer for Gene Therapies
By Amy Bernstein | Friday, May 23, 2025
5 Second Summary
Ultra-rare doesn’t mean unimportant. Kickstart is tackling the unique challenges of developing gene therapies for ultra-rare neuromuscular diseases — bridging the gap from research to real treatments.
All of the more than 300 neuromuscular diseases MDA covers are rare. However, within many of these disorders, there are dozens of subtypes, often characterized by the genetic mutations that cause them. Many specific diagnoses in the neuromuscular community are ultra-rare, affecting fewer than 1 in 50,000 people in the United States.
This presents unique challenges for the researchers and biopharmaceutical companies that develop new therapies:
- An ultra-rare disease may not have been studied enough for scientists to understand how it works in the body and how symptoms typically progress. This knowledge is crucial for designing clinical trials and measuring the effectiveness of a new therapy.
- Rarity makes it difficult to recruit patients for studies and clinical trials. It also adds expense if patients scattered across the country, or even the world, must be brought to a location to administer a therapy or conduct testing.
- Fewer people with a disease means the market for a new therapy will be small. However, the cost of developing a drug is the same whether the market is large or small.

Sharon Hesterlee, Ph.D., is MDA’s interim President and CEO
Many drugmakers consider it too risky to invest in developing a drug if they might not make their money back through sales. That’s why MDA started the Kickstart for Ultra-Rare Neuromuscular Diseases program. By partnering with academic institutions, industry leaders, and the broader community, Kickstart is working to bridge the gap between early scientific research and the development of gene therapies for neuromuscular diseases.
“This is a bold new venture designed to accelerate the development of gene therapies for conditions so rare that they often fall under the radar of traditional drug development efforts,” says Sharon Hesterlee, PhD, MDA’s interim President and CEO.
Kickstart came about from listening to members of the ultra-rare disease community who often feel overlooked by the industry. In late 2024, Kickstart launched a pilot project to develop a gene therapy for a type of congenital myasthenic syndrome (CMS) that affects about 200 American children and adults.
What is Kickstart?
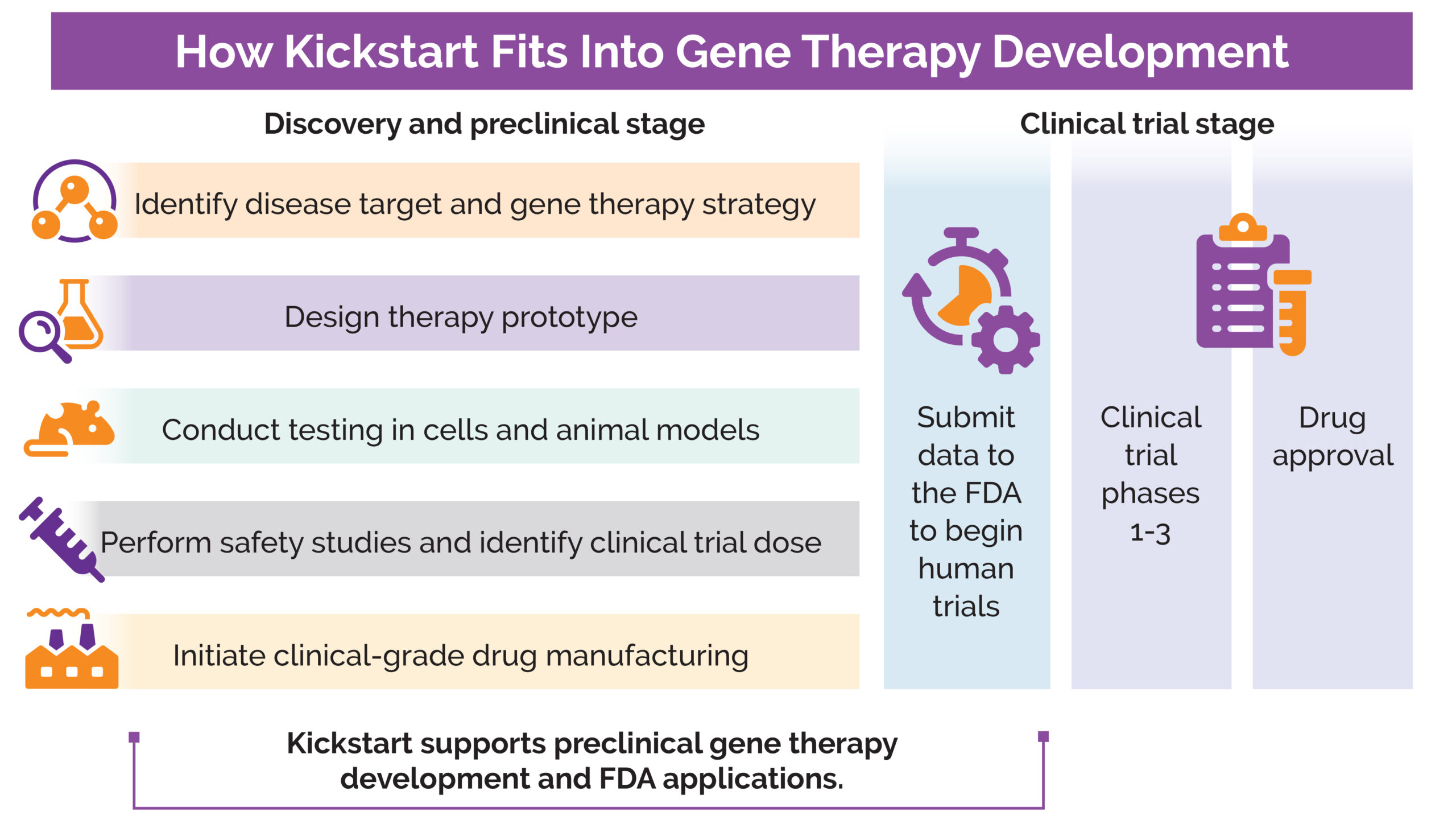
The gene therapy development process is long and involves several steps during the discovery and preclinical stages before a drug is tested in humans in clinical trials. (Learn more about drug development in What Is the Drug Development Pipeline?) The process often starts with early research in an academic lab. If the research shows promise, industry partners, including biopharmaceutical companies, generally step in with the funding and knowledge to help prepare for clinical trials, initiate drug manufacturing, and navigate the US Food and Drug Administration (FDA) review process. All too often, treatments for rare diseases don’t attract the attention of biopharmaceutical companies.
With Kickstart, MDA aims to solve some of the challenges that prevent ultra-rare disease research projects from moving beyond preclinical stages and make them less risky for industry partners to support.

Angela Lek, PhD, is MDA’s interim Chief Research Officer
As an umbrella organization that has backed groundbreaking research across neuromuscular diseases for decades, MDA is uniquely positioned to bridge the gap between early scientific research and the development of gene therapies.
“Developing a gene therapy involves going through a long drug development process, aligning incentives for all stakeholders, and dealing with a lot of things beyond the science,” says Angela Lek, PhD, MDA’s interim Chief Research Officer. “We’re thinking about the entire landscape and ultimately how to get this drug to the patients.”
Kickstart’s initial focus is adeno-associated virus (AAV) gene replacement therapy, which delivers a healthy copy of a gene to replace or do the job of a mutated gene.
“The path of drug development for AAV gene replacement therapy is pretty well mapped out, and there’s precedence for approval of gene therapy drugs in neuromuscular diseases,” says Dr. Lek, referring to Zolgensma® for spinal muscular atrophy (SMA) and Elevidys® for Duchenne muscular dystrophy (DMD). “If you select the right disease that’s amenable to gene replacement therapy, you can potentially make a big impact in patients’ lives with this technology.”
Pilot project
The first research project in MDA’s Kickstart program focuses on developing a gene therapy for CMS caused by choline acetyltransferase (ChAT) gene mutations.
The ChAT gene provides instructions for making a protein that the body needs to transmit electrical signals from nerves to muscles. A ChAT gene mutation disrupts these electrical signals, resulting in low muscle tone, paralysis, and apnea (pauses in breathing) in infancy, sometimes leading to death or brain damage caused by oxygen loss.
“This is a rare disease that doesn’t have any interest from industry, but the disease has a big impact,” says Ricardo A. Maselli, MD, Professor of Neurology and Clinical

Ricardo A. Maselli, MD, is working with Kickstart to develop a new gene therapy.
Neuroscience at the University of California (UC) Davis and a leading expert on CMS.
Dr. Maselli first realized that AAV gene therapy might work in CMS in 2017, when he attended a research presentation at UC Davis. “The researcher described a project that tackles spinal muscular atrophy by carrying a healthy gene in an adeno-associated virus that has a predilection to go to the motor neurons,” Dr. Maselli says. “All of a sudden, it dawned on me that if that could work on spinal muscular atrophy, it could work in other conditions in which the translation of the protein is occurring in the motor neuron.”
At the time, he didn’t know that the research he had learned about would lead to the approval of Zolgensma in 2019, but he began looking for a form of CMS that would be amenable to AAV gene replacement therapy. He obtained funding to create a mouse model of ChAT-related CMS, and although he achieved promising results with the mice he treated, large companies weren’t interested in helping him take his research further. “They care about the revenue and how common the disease is. Congenital myasthenic syndromes are rare, and this subtype is ultra-rare,” he says.
This is where MDA’s Kickstart program came in. For their first project, the Kickstart team wanted to focus on a disease with a combination of great need, low industry interest, and a viable pathway to success and approval. Dr. Maselli’s project was the perfect fit.
Currently, Dr. Maselli is studying four different AAV prototypes in mouse models. “We want to see which one is the best performing,” he says. When a lead candidate is selected, he’ll work on refining the therapy’s effectiveness.
MDA’s Kickstart team acts as a true partner in this process. They meet with Dr. Maselli weekly, bring in other researchers to consult, and have established a partnership with Forge Biologics to manufacture the gene therapy drug for initial testing. MDA also manages communications with the FDA. In October 2024, MDA announced that the project received Rare Pediatric Disease and Orphan Drug designations from the FDA, which provide incentives to develop drugs for rare diseases and may help attract other industry partners.
“I think it’s a huge success for us,” says Marina Kolocha, PhD, PharmD, Kickstart Project Manager. “These two designations mean that the FDA accepts how we define the disease, our estimate of patients, and the emergency of unmet need.”
Dr. Maselli hopes that by the end of the year, the team will begin talking with the FDA about how to transition the gene therapy from animals to human clinical trials.
An innovative model
The pilot project has taken significant steps toward developing an effective therapy for ChAT-related CMS, but Kickstart’s ultimate goal is larger.
“With this pilot program, we are forming due diligence, strategic, and operational practices for translating from academia and nonprofit to patients for access and use,” Dr. Kolocha says. “Along the way, we’re looking to share lessons learned and partner with other nonprofits that want to act as drug developers.”
Dr. Kolocha points out that it is beneficial to have nonprofit organizations involved in drug development because they are just as concerned with the human impact as the science.
“For most of us working with ultra-rare diseases, it’s personal,” she says. “We either have a relative or we are affected with ultra-rare conditions. We know what it means to fight for yourself and for loved ones.”
“We would like to be a model,” Dr. Maselli says. “Kickstart is very innovative. If this works for MDA, it can be moved to other types of organizations. We can show the rest of the scientific community that agencies and researchers can be successful partners.”
This model holds great promise for hundreds more ultra-rare diseases that could be treated with gene therapy.
“Our goal is to ensure that even those with the rarest conditions have a chance to benefit from the incredible advancements being made in genetic medicine,” Dr. Hesterlee says.
Amy Bernstein is an editor and writer for Quest Media.
Gene Therapy Resources
News about gene therapies can be exciting and overwhelming. MDA offers several resources to help individuals and families navigate the changing neuromuscular disease treatment landscape.
Access to Gene Therapy Workshop: Take this online workshop to learn about common considerations before, during, and after gene therapy treatment.
Gene Therapy Community Support Group: This monthly online meeting welcomes parents or guardians of kids eligible for or already treated with an FDA-approved gene therapy.
Gene Therapy Support Network: Gene Therapy Support staff are available to answer questions by phone, email, or video call. Contact them through the MDA Resource Center at 833-ASK-MDA1 or ResourceCenter@mdaUSA.org.
Print-ready Education Materials: Download fact sheets, checklists, and other materials on gene therapy topics.
Next Steps and Useful Resources
- Keep up-to-date on MDA’s Kickstart program.
- Learn more about congenital myasthenic syndrome causes and treatments.
- Read What About My Disease? to learn how researchers are working to translate advances in treatments across diseases, even those considered ultra-rare.
- When parents, advocates, and healthcare providers in the neuromuscular community work together, we accomplish amazing things. Listen to the story behind the life-saving PJ’s Protocol.
- Stay up to date on Quest content! Subscribe to Quest Magazine and Newsletter.
Disclaimer: No content on this site should ever be used as a substitute for direct medical advice from your doctor or other qualified clinician.




